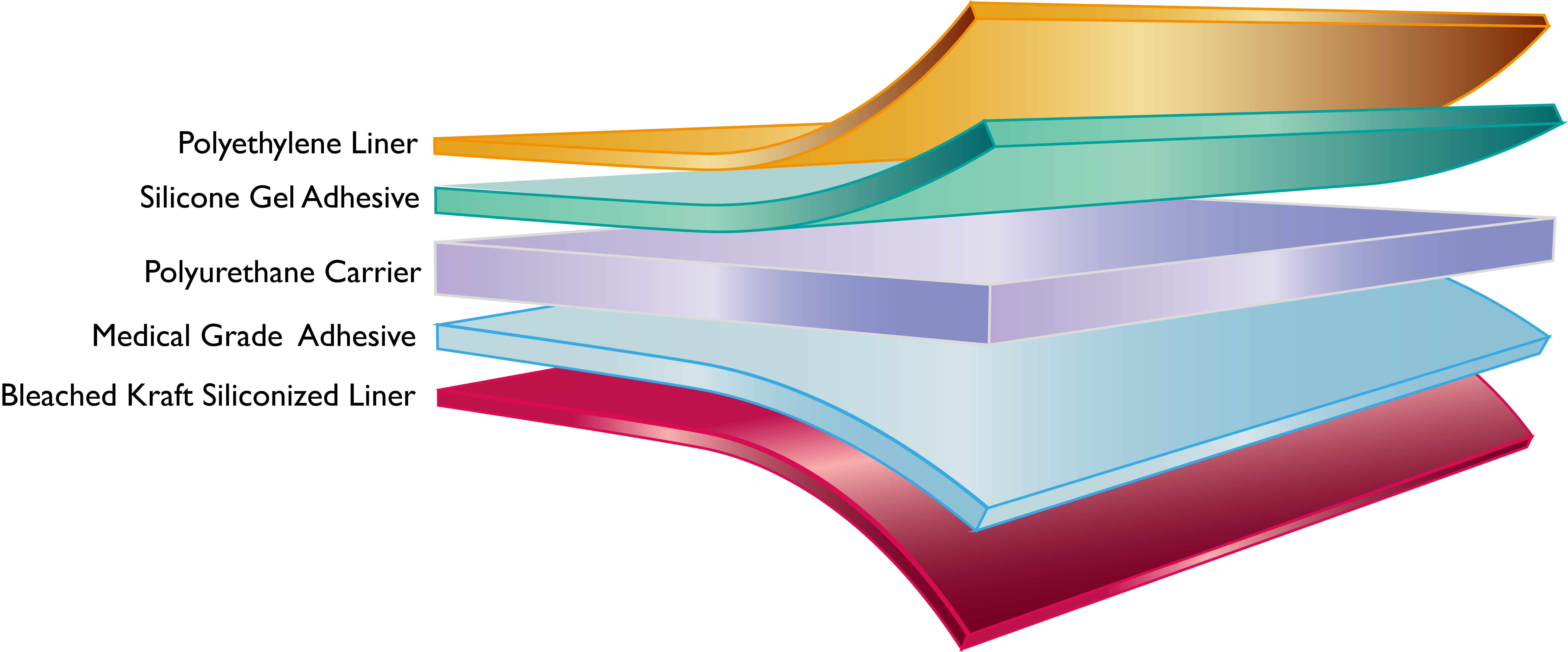
P-DERM® PS-1835 is a trilaminate consisting of high adhesion silicone gel adhesive, polyurethane film and medical grade adhesive where consistent adhesion and repositionability is required. PS-1835 is supplied with a 100 micron polyethylene liner on the silicone gel adhesive side and 54# bleached kraft siliconized liner on the medical grade adhesive side of the construction.
P-DERM
PS-1835
• Excellent Instantaneous Tack
• Atraumatic Removal from Skin
• Hypoallergenic
• Ability to be Removed and Repositioned
• Excellent Converting Properties
• Atraumatic Removal from Skin
• Hypoallergenic
• Ability to be Removed and Repositioned
• Excellent Converting Properties
| Property | Value | Test Method |
|---|---|---|
| Medical Grade Adhesive Adhesion | >10 N/25mm | QSP-722 |
| Adhesive Coat Weight | 37 gsm | QSP-724 |
| Recommended Sterilization Method | Ethylene Oxide | -- |
| Continuous Use Conditions | 10 - 70 C | QSP-754 |
| Biocompatibility | Cytotoxicity: Pass; Primary Dermal: Pass; Buehler Sensitization: Pass | ISO 10993 |
| Silicone Adhesion | 1.7 N/25mm | QSP-723 |
| Carrier Thickness | 25 micron | QSP-726 |
| Carrier Type | Polyurethane Film | -- |
| Silicone Gel Coat Weight | 150 gsm | QSP-724 |
| Thickness (excluding liners) | 0.22mm | QSP-726 |
Specific tests should be performed by the end user to determine the product stability for the particular application.
