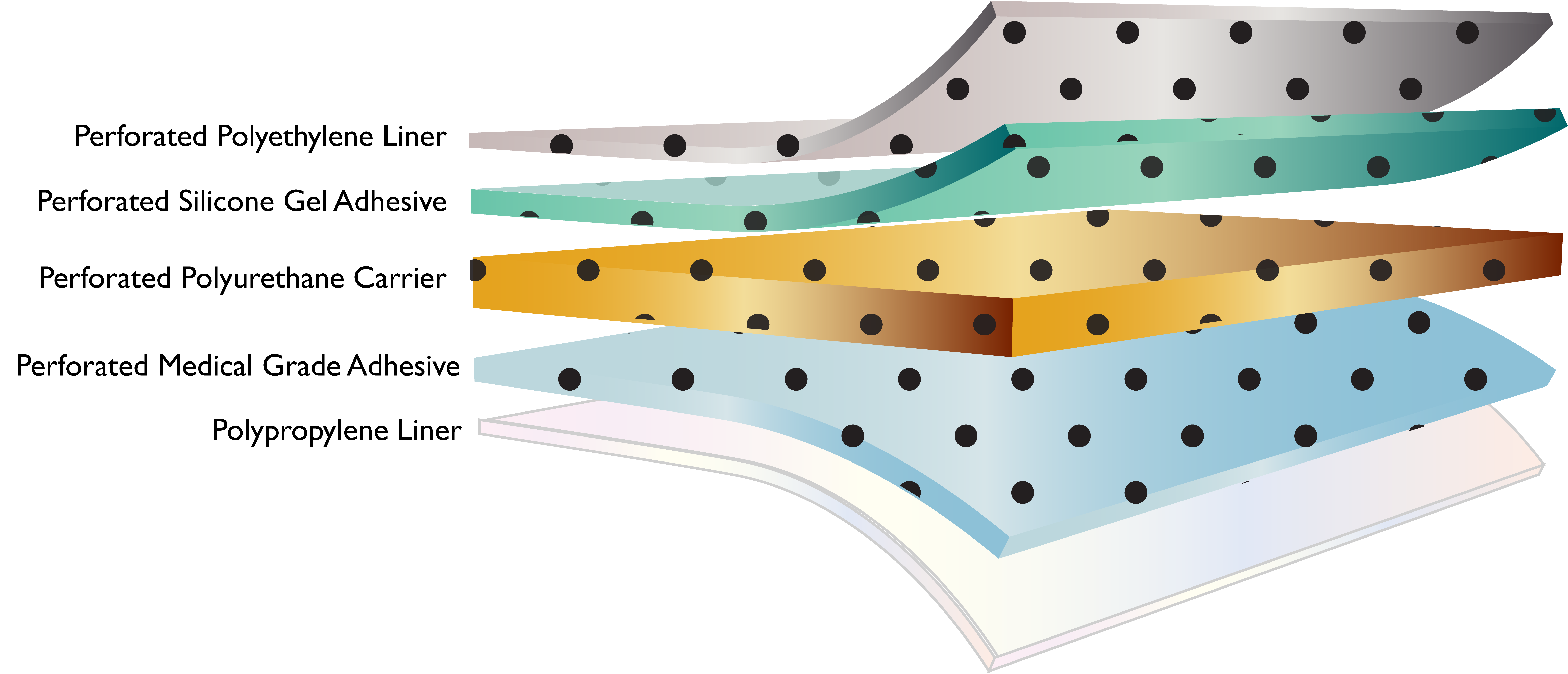
P-DERM® PS-1836 is a trilaminate consisting of perforated high adhesion silicone gel adhesive, polyurethane film carrier and medical grade adhesive where consistent adhesion and repositionability is required. PS-1836 is supplied with a perforated 100 micron polyethylene liner on the silicone gel adhesive side and non-perforated white polypropylene siliconized liner on the medical grade adhesive side of the construction.
P-DERM
PS-1836
Hypoallergenic
Ability to be Removed and Repositioned
Atraumatic Removal from Skin
Excellent Instantaneous Tack
Excellent Converting Properties
Ability to be Removed and Repositioned
Atraumatic Removal from Skin
Excellent Instantaneous Tack
Excellent Converting Properties
| Property | Value | Test Method |
|---|---|---|
| Perforation Hole Size/Open Area | 1.5mm / 17% | -- |
| Medical Grade Adhesive Adhesion | >8 N/25mm | QSP-722 |
| Adhesive Coat Weight | 37 gsm | QSP-724 |
| Recommended Sterilization Method | Ethylene Oxide | -- |
| Continuous Use Conditions | 10 - 70 C | QSP-754 |
| Biocompatibility | Cytotoxicity: Pass; Primary Dermal: Pass | ISO 10993 |
| Silicone Adhesion | 1.4 N/25mm | QSP-723 |
| Carrier Thickness | 25 micron | QSP-726 |
| Carrier Type | Polyurethane Film | -- |
| Silicone Gel Coat Weight | 150 gsm | QSP-724 |
| Thickness (excluding liners) | 0.22mm | QSP-726 |
