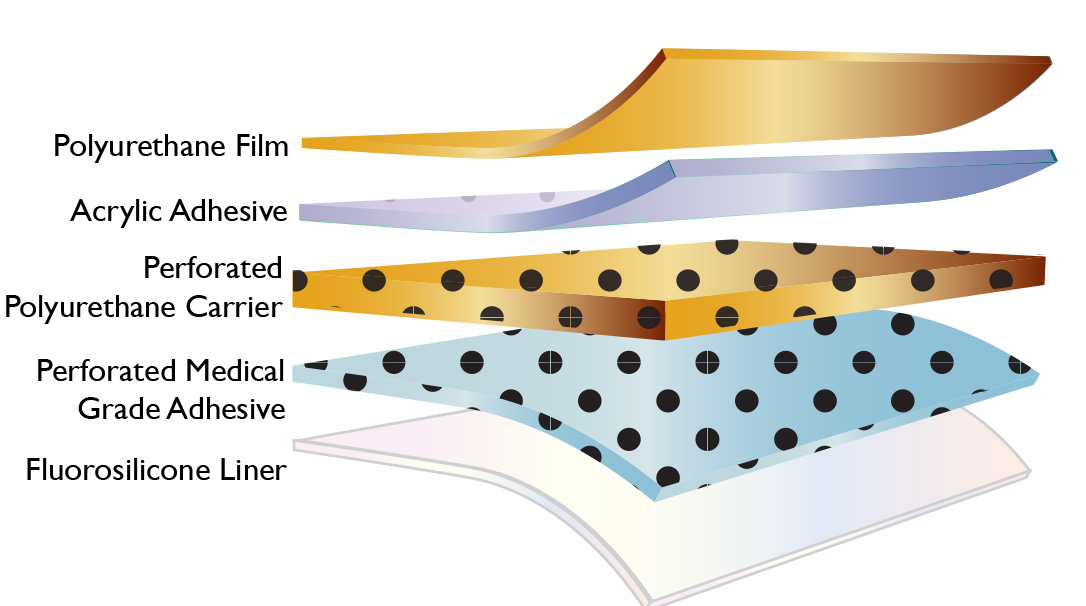
P-DERM® PS-1883 is a hybrid silicone/acrylic perforated bilaminate consisting of perforated high adhesion silicone gel adhesive, perforated polyurethane film and non-perforated acrylic adhesive and polyurethane face. PS-1883 provides the low trauma removal from a silicone gel, but improved shear resistance, skin anhorage and breathability of an acrylic. A fluorosilicone liner is used to provide compatibility with the silicone gel and acrylic adhesives.
P-DERM®
PS-1883
• Excellent Instantaneous Tack
• Good Resistance to Shear
• Atraumatic Removal from Skin
• Excellent Breathability
• Hypoallergenic
• Ability to be Removed and Repositioned
• Excellent Converting Properties
• Good Resistance to Shear
• Atraumatic Removal from Skin
• Excellent Breathability
• Hypoallergenic
• Ability to be Removed and Repositioned
• Excellent Converting Properties
| Property | Value | Test Method |
|---|---|---|
| Thickness (excluding liners) | 0.24mm | QSP-726 |
| Silicone Gel Coat Weight | 150 gsm +/- 25gsm | QSP-724 |
| Carrier Type | Polyurethane | -- |
| Carrier Thickness | 25 micron (x2) | QSP-724 |
| Acrylic Coat Weight | 38 +/- 5gsm | QSP-724 |
| Adhesion | 3.5 N/25mm | QSP-724 |
| Moisture Vapor Transmission Rate (upright) | 38 +/- 5gsm | QSP-727 |
| Continuous Use Conditions | 10 - 70 C | QSP-754 |
| Biocompatibility | Cytotoxicity: Pass Primary Dermal: Pass Buehler Sensitization: Pass | ISO 10993 |
| Recommended Sterilization Method | Ethylene Oxide | -- |
Specific tests should be performed by the end user to determine the product suitability for the particular application.
